Chapter-12 Radioactivity ICSE
Radioactivity Class 10 and 12 Notes Physics and Important question
Intrduction
An atom has electrons, protons and neutrons. The protons and
neutrons are present in the nucleus and are called nucleons while the electrons
revolve around them in specific orbits or shells. The number of shells in an
atom depends upon the number of electrons it has. The maximum number of
electrons a shell can hold is determined by the formula 2n2, where n
is the shell number. The shells are also named K, L, M, N, O, P, and Q.
The size of an atom is of the order 10-10 m. An
electron has a negative charge of -1.6 X 10-19 C. Its mass is nearly
9.1 X 10-31 kg.
The nucleus of an atom is about 10-15 to 10-14
m. Protons have a charge of +1.6 X 10-19 C. Its mass is 1.67 X 10-27
kg. Neutrons are electrically neutral particles. Its mass is also the same as
that of a proton. They are collectively called nucleons. The nucleus is
positively charged.
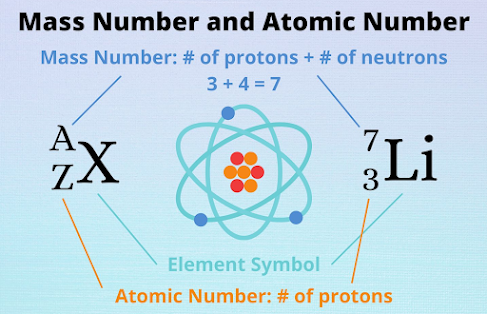
Atomic number (Z): The atomic number of an atom is equal to
the number of protons in its nucleus.
Mass number (A): The mass number of an atom is equal to the
total number of nucleons in its nucleus.
Since an atom is electrically neutral, number of protons is
equal to the number of electrons. An atom is specified as XAz.
Chemical change: Change in the number of electrons
Nuclear change: Change in the number of protons or neutrons
1 eV = 1.6 X 10-19 V. Chemical changes needs
energy in the order of a few eV. Nuclear changes need energy in the order of a
few MeV.
In nuclear reactions, the total number of electrons and
protons remain conserved.
Isotopes are atoms of the same element, having same atomic number
but different mass number. This is due to difference in the number of neutrons.
Their chemical properties are also same. E.g. Protium, Deutrium.
Tin (Sn) has the largest number of isotopes (10).
Stable isotopes are those that have the number of neutrons
nearly equal to the number of protons. Unstable or radioactive isotopes are
those that have more neutrons than protons in the nucleus.
Isobars are the atoms of different elements which have the
same mass number but different atomic number. E.g. Na-23, Mg-23.
If the number of protons and neutrons get interchanged
inside the nucleus in the isobars, they are called mirror isobars.
Isotones are atoms having different number of protons but
same number of neutrons. They have different number of electrons. E.g. K-39,
Ca-40.
Henry Becquerel discovered the phenomenon of radioactivity
in 1896. He left a uranium salt in black paper placed on a photographic plate.
After some days, he found that the photographic plate had been affected.
Similar results came with other salts of uranium. These radiations could pass
through black paper, glass or wood. These radiations were called Becquerel
rays. These rays were of three types 1) positively charged (alpha) 2)
negatively charged (beta) 3) uncharged (gamma) rays. The substances which emit
radiation are called radioactive substances.
The substances which disintegrate by the spontaneous
emission of radiations, are called radioactive substances.
All the elements which have atomic number more than 82 are
naturally radioactive substances.
Any physical or chemical changes does not change the nature
of radiation emitted by the substance and its rate of decay. Therefore, it is
not due to the electrons which are affected by this. Radioactivity is the
property of the nucleus.
Radioactivity is the nuclear process of spontaneous emission
of alpha, beta, and gamma radiations from the nucleus of atoms during their
decay.
There is no way to know which atom of the element would
decay since it is a random phenomenon.
In 1903, Rutherford found that when the radiations emitted
by the radioactive substances are subjected to a perpendicular magnetic field,
they separate into three distinct rays. He found that one of the rays was
positive (Alpha), one was negative (beta) and the undeflected rays (gamma). The
beta rays were deflected more than the alpha rays. Similar results came when
these rays are subjected to an electric field. Always, the beta rays are deflected
more than the alpha rays due to less mass.
Properties of alpha particles:
- · It is a helium nucleus containing two protons and neutrons.
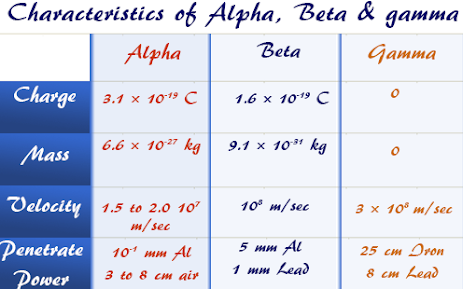
- · Mass: 6.68 X 10-27 kg, Charge: 3.2 X 10-19 C, specific charge: 4.79 X 107 C kg-1
- · Speed is of the order of 107 m s-1. Alpha particles emitted from different substances and even in the same substance differ in speed. Their energy is distributed in a small range.
- · It strongly ionizes the gas through which it passes. It ionizing power is 100 times that of beta particles and 10,000 times that of gamma particles.
- · As it dissipates energy rapidly, its penetrating power is very small (only about 3-8 cm in air). It can easily be stopped by a card or thick paper.
- · It is deflected in a magnetic or electric field since it is positively charged. It deflection is small as that of a beta particle since it is more massive.
- · They affect a photographic plate.
- · They cause fluorescence on striking a fluorescent material.
- · They have high kinetic energy and momentum with them, Hence, they are used for bombarding elements for transmutation.
- · They destroy living cells and cause biological damage.
- · They get scattered when passing through thin mica or gold foils.
Properties of beta rays:
- · They are electrons.
- · Mass: 9.1 X 10-31 kg, Charge: -1.6 X 10-19 C, specific charge: -1.76 X 1011 C kg-1
- · They are given out of the nucleus of the atom.
- · Speed: less than 3 X 108 m s-1. Different beta particles emitted from the same substance differ in speeds.
- · Ionising power is roughly 1/100 times that of alpha particles and 100 times that of gamma radiations.
- · They have more penetrating power than alpha particles. They can travel 5 m in air. They can be stopped with a 5 mm thick aluminum sheet.
- · Beta particles are deflected by the magnetic and electric fields because they are negatively charged. They are deflected more than the alpha particles since they are light.
- · They affect a photographic plate.
- · They produce fluorescence striking a fluorescent material.
- · They produce X-Rays when they are passed through metals of high atomic number and melting point like tungsten.
- · They cause more biological damage than alpha particles because they can easily pass through the skin of the body.
Properties of gamma radiations:
- · They are electromagnetic radiations like X-Rays and light with a wavelength of 10-4 nm
- · Speed: 3 X 108 m s-1
- · Ionising power is very low.
- · The penetrating power is very high. It is about 10,000 times that of alpha particles. It can pass 500 m in air and 30 cm in an iron sheet. A lead sheet can stop them.
- · They are not deflected by the electric and magnetic fields.
- · They affect a photographic plate.
- · They cause fluorescence when striking a fluorescent material.
- · They can be diffracted by X-Rays.
- · They are given out of the nucleus unlike X-Rays.
- · They cause immense biological damage as they can pass through the human body.
- · They are useful in the treatment of cancer.
The gamma radiations are the most energetic and suffer least
collisions and therefore have the least ionising power and the most penetrating
power. The alpha particles are the least energetic and suffer most collisions
and therefore have the most ionising power and least penetrating power.
Radioactivity is a nuclear phenomenon. Alpha, beta and gamma
radiations are given out to form a more stable nucleus. In the emission of
alpha and beta rays, there is a change in the number of protons and neutrons
while in the emission of gamma rays, there isn’t.
Alpha emission: In a radioactive element, two protons and
two neutrons tightly bound together are released from the nucleus. These are
called alpha particles and the stream is called alpha rays. Mass number
decreases by 4 and atomic number decreases by 2 from the parent nucleus to form
the daughter nucleus in the atom for the emission of a single alpha particle.
Beta emission: In an unstable atom, neutrons may split into
protons and electrons. These electrons are repelled away from the nucleus at
high speeds. The particles are called beta particles and the rays are called
beta rays. To keep the number of particles either odd or even on both sides, an
antineutrino (uncharged, massless) particle is also assumed to be emitted along
with the beta particle. In the emission of a beta particle, the mass number of
the nucleus of the parent atom and the daughter nucleus remains same but the
atomic number increases by one.
Gamma radiation: When the daughter nucleus of an atom formed
after the emission of an alpha or a beta particle has excess energy, the energy
is released by an electromagnetic radiation known as gamma radiation. There is
no change in the mass or atomic number after the radiation. This radiation continues
till the atom comes in a ground state.
Beta particles are electrons that are created in the nucleus
of a radioactive atom.
Alpha and beta radiations are never together, but they may
be accompanied by gamma radiations.
The daughter nucleus formed after radiation still undergoes
radioactivity if it is not stable.
Continuation Part 2 is here.......



0 Comments
Hope Everyone Reading my posts are gaining KNOWLEDGE and able to know something new and informative.
📚📖📕🧾📝😅
Sharing is Caring. So please share this website with everyone you know so that they can also improve their KNOWLEDGE !!!