Atomic structure and chemical bonding
Introduction
Many attempts have been made to know as to what is the ultimate particle of matter.
The idea of smallest unit of matter was the first given by Maharashi kanada in the 6th century B.C.in India.
According to him matter consisted of minute particles called as paramanus (param means ultimate and anu means particle)now called as atoms.
Paramanus does not exist in free state, it will come with other paramanus a bigger particle called anu now know as molecule .
Definition of an element
element is a pure substance that can neither be formed from,nor decomposed into simple substance by ordinary physical or chemical methods.
For example Carbon is an element because it cannot split into two or more simple substance by ordinary methods like heating,breaking or passing electricity.
Note:Radioactivity is the processes of radioactive decay and high energy nuclear reaction can transform one elementary substance into another.
Definition of an atom
"An atom is the smallest particle of an element that exhibits all the properties of that element. I may or may not exist independently but takes part in every chemical reaction".
Example :
Take a small pieces of zinc and break into simpler pieces.
All these pieces shows the properties of zinc.
On grinding them further they will break up into very fine particles which still show the properties of zinc but there are a stage when the particle cannot further subdivided into particles cannot be further subdivided into particles exhibiting properties of zinc.
These invisible particles are the atoms of zinc.
Discovery of Electrons
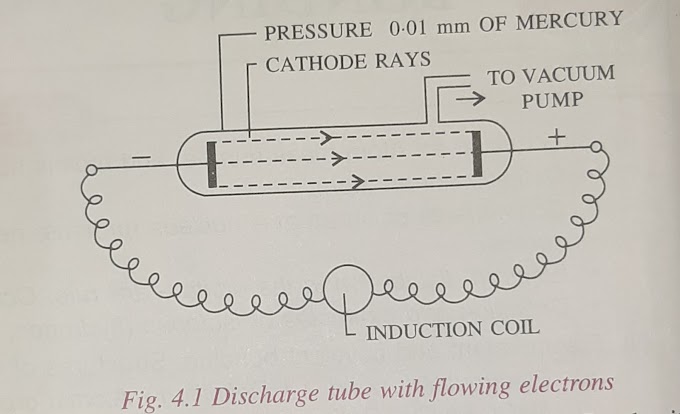
William crooke , a British scientist noted that gases are poor conductor when a high voltage of 10000 volts charge from an induction coil is applied to tubes filled with gases become good conductors of electricity and begin to flow From cathode to anode in the form of rays.
Since these rays originates from the negative plate,that is the cathode and travel from the cathode towards the anode,they are called cathode rays.
later Jj thomson Studied the characteristics and the constituents of cathode rays.
The apparatus used by him is called a discharge tube or a cathode ray used by him is called a discharge tube or a cathode ray.
A discharge tube is a hard glass fitted with two metal plates known as electrodes one of which is connected to the negative terminal of the battery is called cathode.
It has a side tube through which gas can be pumped out by using a vacuum pump to crate a vacuum
When Electrical discharge of 10000 volts is passed through gases at very low pressures (0.01mm),cathode rays are produced.
Properties of cathode rays
- They travel from cathode to anode
- They cause a greenish yellow fluorescence on a soda-glass screen placed in the tube.
- They penetrate through matter.
- They cause ionization of the gas through which they pass.
Thomson concluded that
- Cathode rays consist of negatively charged particles, now called electrons.
- these negatively-charged particles are an integral parts of all atoms.
- electrons have both definite mass and definite electric charge, both of which are independent of the nature of the gas in the discharge tube.
Properties of electrons
- Electrons from all source are a like having identical mass.
- They are a constituent part of all atoms.
Discovery of protons
Goldstein noticed that another set of rays travelling in a direction opposite to that of the cathode rays that is from anode towards cathode.
He called these rays as a canal rays these rays passed through holes or canals in the cathode .
These rays were named as positive rays or anode rays.

Properties of Anode rays
- Anode rays travel in a straight line.
- They are made up of positively charged particles.
Properties of protons
- A proton possesses a unit positive charge +1 of the value 1.602x10-19
- coulombs.
- Its mass is the same as that of a hydrogen atom
- The proton resides in the central part of an atom,i.e. in the nucleus.
Discover of neutrons
We know that atom contains electrons and protons and that the atomic mass of an electron is negligible.
therefore,an atom of helium,which contains 2 protons should have a mass = 2x1a.m.u. but the atomic mass of a helium atom was found to be a approximately 4.0 a.m.u
It was therefore,proposed that in the nucleus of an atom,there must be another particle.this particles should not posses any electrical charge and must be equal in mass to the proton.
A neutron is a sub-atomic particles or fundamental particle of an atom with no charge and mass almost equal to the mass of the proton i.e. hydrogen atom.
Neutron is donated by 0n1.
The superscript 1 represent its mass and subscript 0 represents its electrical charge.
Properties of neutrons
- This particle was not found to be deflected by any magnetic or electric field, proving that it is electrically neutral.
- Its mass is equal to 1.676x10-24g(1 amu)
Atomic Number Z
- An atom of an element has its own character number of protons in its nucleus, which distinguishes it from the atoms of other elements.
- This character number is distinguish from the atom of other element.
- Atomic number is denoted by Z.
- The atomic number is therefore equal to the number of electrons in the neutral atom of an element.
No .of protons =no.of electrons
- Number of protons gives the total positive charge present in the nucleus of an atom. Atomic number is unique for atoms of each element.
The atomic number of element is the number of :
- electrons present in its neutral atom.
- protons presents in the nucleus of its atom.
- positive charge in the nucleus of its atom.
Valence electrons
The outermost shell of an atom is called its valence shell, and the electrons present in the valence shell are known as valence shell are known as valence electrons.
Hydrogen has only one electron. Similarly, carbon has one shell and so it has 4 valence electrons.
The chemical properties of elements are decided by these valence are electrons, since they are the ones that take part in chemical reactions.
The elements with the same number of electrons in the valence shell show similar properties ;those with different number of valence electrons show different chemical properties.
Example of isotopes
Element number of isotopes
1.Hydrogen Three 1H,2H,3H
1 1 1
2.Carbon Three 12C,13C,14C
6 6 6
3.Chlorine Two 35CL,37CL,
17 17
4.Oxygen Two 16O,18O
8 8
5.Potassium Two 39K,41K
19 19
Properties of isotopes
- Isotopes have similar chemical properties.
- Isotopes have same number.
- The same number of electrons and protons and hence the same electronic configuration.
- Chemical properties and determined by the electronic configuration of an atom.
- Isotopes of an element are chemically alike.
- Physical constants or physical properties depend on the atomic mass
- Isotopes differ in few physical properties such as density,boiling point,etc.
- physical constant or physical properties depend on the atomic mass (mass number)and isotopes have different mass numbers (A) due to different number of neutrons.
Uses of isotopes
1.Isotope is cobalt 30Co is used in radiotherapy for treating cancer and
27
other disease
2.Radioactive isotopes are used in industry to detect the leakage in underground oil pipelines,gas pipelines and water pipes.
Covalent bond is formed when both the combining athoms have four or more electrons in their outermost shells,i.e., non metals (exceptions are H,be,B,etc.).
Also read this "Geographic grid"




2 Comments
Great post for beginners In chemistry😁😁😁
ReplyDeleteGood one for all beginners in chemistry
ReplyDeleteHope Everyone Reading my posts are gaining KNOWLEDGE and able to know something new and informative.
📚📖📕🧾📝😅
Sharing is Caring. So please share this website with everyone you know so that they can also improve their KNOWLEDGE !!!