Radioactivity Physics
Radioactivity Lesson Important question in Nuclear Fission and Fusion and Uses of fission
Background radiations:
Background radiations are radioactive radiations to which we are all exposed even in the absence of actual radioactive sources.
There are two sources of background radiations:
- Internal sources: They are present inside our body like K-40, C-14 (These affect all parts equally) and radium (They affect bones and reproductive organs).
- External sources: They are outside sources like cosmic rays, solar radiations and radiations from radioactive elements.
It is not possible for us to keep ourselves away from background radiations. They do not exceed the maximum harmful limit and does not affect our safety.
The effect of radiations in human tissues are measured in a unit called Sieverts (Sv) dose.
Nuclear Fission and Fusion:
Due to radioactive phenomenon like decay, fission or fusion, mass is reduced from the parent nucleus to form the daughter nucleus. The energy released due to the loss in mass is found by the formula given by Einstein in 1905.
The formula for energy released is E = mc2 where m is the loss in mass an c is the speed of light. This energy is called nuclear energy.
The energy released for the loss in mass of 1 kg is 9 X 1016 J.
1 kWh = 3.6 X 106 J.
This energy is equivalent to 2.5 X 1010 kWh
1 a.m.u. = 1.66 X 10-27 kg
The energy released due to loss of one a.m.u. is 931 MeV
The energy released by the subatomic particles are given below:
- Electron: 0.511 MeV
- Proton: 938.3 MeV
- Neutron: 939 MeV
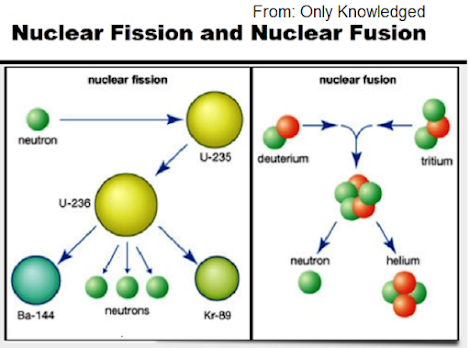
Nuclear fission is the process in which a heavy nucleus splits into two light nuclei of nearly the same size, when bombarded with slow neutrons to release a tremendous amount of energy.
Energy is released because the sum of mass of product nuclei is less than the mass of parent nucleus and of neutron. The loss in mass is converted into energy.
In 1939, Strassman and Otto-Hann found that when a slow neutron strikes a U-235 nucleus, the most unstable nucleus U-236 is formed. U-236 splits into Ba-144 and Kr-89 and releases three neutrons.
U-236 isotope can also decay into: 1. Ba-141 and Kr-92, 2. La-148 and Br-85.
In nuclear fission, atomic and mass numbers are conserved.
The nuclei produced by U-236 are unstable and they emit beta and gamma rays.
The sum of mass of daughter nuclei and neutrons is less than the sum of masses of parent nucleus and the neutron.
Loss in mass in the above fission reaction is 0.20 a.m.u.
Thus, energy released by the loss in mass = 931 X 0.20 = 190 MeV.
The energy obtained is mainly in the form of kinetic energy of the daughter nuclei and the remaining part by the kinetic energy of the released neutrons, gamma rays, heat and light.By Fission of 1g of U-235 nuclei (2.56 X 1021), nearly 4.8 X 1023 MeV energy is released. 2.1 X 104 kWh electrical energy can be obtained. This is the energy liberated by 20 tonnes of TNT (trinitrotoluene).
Uranium ores found in nature has 99.3% U-238 and 0.7% U-235. U-235 is more easily fissionable than U-238 because U-238 needs fast neutrons for fission.
Each U-235 atom when bombarded with a slow neutron, it splits into two nuclei (Ba-144 and Kr-89) and releases three new neutrons. These three neutrons bombard other U-235 nuclei and release energy. This goes on and on is called a chain reaction. They can occur in a very short interval of time and releases energy which are harmful. Those which are not controlled are called uncontrolled chain reactions.
If the chain reaction is controlled by the use of cadmium rods which absorb neutrons and graphite and heavy water which slows down the neutrons, it is called a controlled chain reaction.
Uses of fission:
- Destructive uses: It is used in a nuclear bomb where energy is released and is uncontrolled.
- Constructive uses: In a controlled chain reaction, the amount of fission is lowered and can be used for producing electricity.
Differences between radioactive decay and Nuclear fission:
- Radioactive decay is a spontaneous process whereas nuclear fission is an initiated process.
- Nucleus emits alpha, beta and gamma rays which are not large in radioactive decay. Nucleus emits tremendous amount of energy, two nearly equal nuclei, when bombarded with neutrons.
- The rate of radioactive decay cannot be controlled whereas the rate of nuclear fission can be controlled.
Nuclear fusion is the process in which two light nuclei combine to form a heavy nucleus. A huge amount of energy is released in the process.
Energy is released because the mass of products is less than the mass of the reactants according to the relation E = mc2.
When two deuterium nuclei fuse, 3.3 MeV of energy is released and helium isotope He-3 and a neutron are formed.
He-3 fuses with a deuterium nucleus to form helium nucleus and a proton and 18.3 MeV energy.
Totally, three deuterium nuclei fuse and 21.6 MeV energy is released. Part of this energy is the kinetic energy of the neutron and proton.
Nuclear fusion is not possible in ordinary temperature and pressure because electrostatic forces of attraction are present between the same charges in the nuclei causing repulsion. They need high temperature (107 K) and pressure to gain sufficient kinetic energy and overcome the repulsive forces and fuse. This is why fusion reaction is also called thermo-nuclear reaction.
Sun and stars emit energy because of the fusion reaction of four hydrogen nuclei with high temperature and pressure to produce a helium atom, 2 positron particles, and 2 neutrino particles. Energy released is of 26.7 MeV.
Working of hydrogen bombs is based on fusion. The energy needed for the reaction is obtained by the bombardment of the nuclear bomb.
The energy released by fusion for a given mass is much more than the energy released by fission for the same mass of heavy nuclei. This is because the number of hydrogen nuclei for fusion in a given mass is more than the number of heavy nuclei used for fission.
Differences between fission and fusion:
- In fission, the nucleus splits into two nuclei of nearly the same size. In fusion, nuclei fuse together with high temperature and pressure.
- Fission is possible in ordinary temperature and pressure whereas fusion takes place in high temperature and pressure.
- 190 MeV energy is released in one fission. Nearly 24.7 MeV energy is released in one fusion.
- For same mass, energy released by fusion is greater than the energy released by fission.
- Fissionable substances are radioactive whereas fusionable substances are not.
- Fissionable substances are limited in nature whereas fusionable substances are found abundantly in nature.
- Fission process can be controlled whereas fusion process cannot be controlled.
- Nuclear bomb is based on fission and hydrogen bombs are based on fusion.




0 Comments
Hope Everyone Reading my posts are gaining KNOWLEDGE and able to know something new and informative.
📚📖📕🧾📝😅
Sharing is Caring. So please share this website with everyone you know so that they can also improve their KNOWLEDGE !!!